Abstract
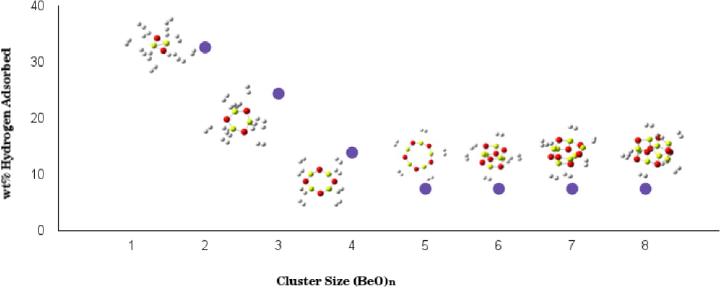
Since the current transportation sector is the largest consumer of oil, and subsequently responsible for major air pollutants, it is inevitable to use alternative renewable sources of energies for vehicular applications. The hydrogen energy seems to be a promising candidate. To explore the possibility of achieving a solid-state high-capacity storage of hydrogen for onboard applications, we have performed first-principles density functional theoretical calculations of hydrogen storage properties of beryllium oxide clusters (BeO)n (n = 2-8). We observed that a polar BeO bond is responsible for H2 adsorption. The problem of cohesion of beryllium atoms does not arise, as they are an integral part of BeO clusters. The (BeO)n (n = 2-8) adsorbs 8-12 H2 molecules with an adsorption energy in the desirable range of reversible hydrogen storage. The gravimetric density of H2 adsorbed on BeO clusters meets the ultimate 7.5 wt % limit, recommended for onboard practical applications. In conclusion, beryllium oxide clusters exhibit a remarkable solid-state hydrogen storage.
Search content by categories